
Product Strategy & Support
Systematic alignment of product decisions with stakeholder needs to maximize project ROI, launch momentum, and rate of adoption
Blending customer perspective, technical expertise, and business strategy
into actionable guidance for the MedTech innovator and investor community
The difference between a promising device and a successful one is simple: adoption in the clinic.
Adoption means solving for both the stakeholders who shape a device’s fate—regulators, adopters, administrators, and payers—and the evidence they demand: safety, efficacy, value, and outcomes.
Within this puzzle of needs and constraints lies the potential for true innovation.
With an integral blend of technical depth and commercial insight, Dabrowiak Consulting helps bring the pieces together.
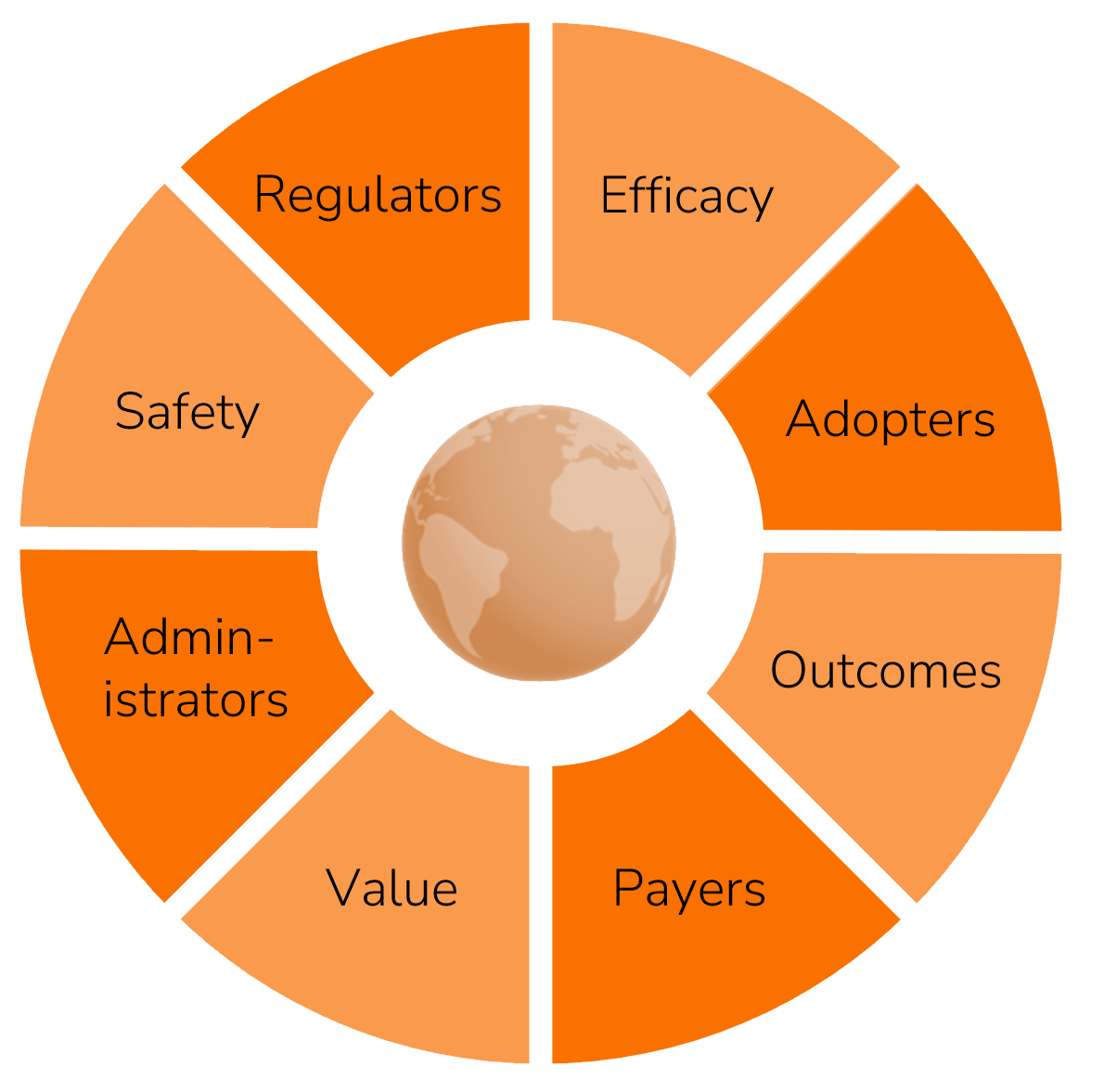

Systematic alignment of product decisions with stakeholder needs to maximize project ROI, launch momentum, and rate of adoption

Unbiased evaluation of technologies and claims to support strategic, intellectual property, and investment decisions
| Customer Research | KOL interviews, user focus groups |
|---|---|
| Clinical process maps (journey, decision, value stream) | |
| Concept Generation | Iterative brainstorming & concept modeling |
| Prioritization matrices (effort, impact) | |
| Feature grouping (fit, overlap) | |
| Product Strategy | Product roadmaps and lifecycle planning |
| Financial models (NPV, ROI) | |
| Product / Marketing Requirements | |
| Development Support | User Interface / User Experience (UI/UX) |
| Actionable VOC feedback | |
| Technical advising | |
| Commercial Support | Positioning, Collateral |
| Product aids (guides, videos) | |
| Sales training & initial customer visits |
| Systems | Architecture, layout |
|---|---|
| Algorithms - measurement, control | |
| Sterile Consumables | Catheters, hub overmolding |
| Cartridges, tubing sets | |
| Fluids | Blood, saline, slurries, supersaturation |
| Valves, traps, dampening, vents | |
| Pumps - roller, gear, vane, centrifugal, gravity, vacuum | |
| Heating & Cooling | System, heat exchanger, and evaporator design |
| Thermoelectrics, desiccation-evaporation, endothermic mtls. | |
| Sensors | Pressure, temperature, flow, bubble, level |
Jeremy Dabrowiak most recently served as Director of Marketing at ZOLL Medical. Prior to that, he served the company in Director-level roles in Innovation and Research, Design Research, and Product Engineering.
In 21 years of startup and corporate medical device roles, he has driven multiple products from concept to commercial launch while co-authoring 38 patents. Prior to medical devices, he worked for 5 years in biotechnology instrumentation, contributing to early work in Next-Generation Sequencing for Lynx Therapeutics (now Illumina).
Jeremy has completed executive courses in innovation, continuous improvement, and engineering management at MIT Sloan and the University of Michigan.
He holds degrees in Mechanical Engineering from Michigan Technological University (B.S. Summa Cum Laude) and Stanford University (M.S.). His 1999 Master's degree project was a Parallel Parking Assistive System for Toyota.

Every success begins with a conversation. Reach out to explore how we can turn complexity into clarity — and ideas into impact.
